Thursday, July 4, 2024 06:35 PM
Triple Therapy Shows Promise in Advanced Gastric Cancer Treatment
- Osemitamab (TST001), Nivolumab, and CAPOX combo demonstrates efficacy in advanced G/GEJ cancer
- High or medium CLDN18.2 expression linked to better treatment outcomes
- Superior PFS benefits observed with triple therapy compared to historical data
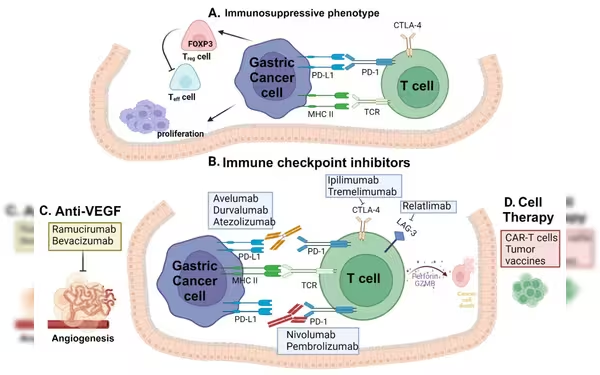 Image Credits: MDPI
Image Credits: MDPIRecent Phase II trial results reveal the efficacy of Osemitamab (TST001), Nivolumab, and CAPOX triple therapy in treating advanced gastric or gastroesophageal junction (G/GEJ) cancer, offering hope for improved outcomes and potentially setting a new standard of care in oncology.
Advanced gastric or gastroesophageal junction (G/GEJ) cancer is a challenging condition that requires effective treatment options. Recent Phase II trial results have shed light on a potential breakthrough in this area, with the combination of Osemitamab (TST001), Nivolumab, and CAPOX showing promising outcomes as a first-line therapy.
The study, known as Cohort G from the TranStar102 trial, focused on patients with advanced G/GEJ cancer, regardless of their CLDN18.2 or PD-L1 expression levels. The results revealed that patients with high or medium CLDN18.2 expression experienced a median progression-free survival (PFS) of 12.6 months, showcasing the efficacy of the triple combination therapy.
Notably, the treatment regimen demonstrated a significant overall response rate of 68% in patients with high or medium CLDN18.2 expression, highlighting its potential as a viable option for individuals with this type of cancer. Furthermore, the analysis indicated a correlation between CLDN18.2 expression levels and anti-tumor efficacy, suggesting that higher expression levels may lead to better treatment outcomes.
Compared to historical data on alternative treatment combinations, Osemitamab (TST001) combined with Nivolumab and CAPOX showed superior PFS benefits, particularly in patients with low PD-L1 expression levels. These findings support the notion that this triple therapy could become a global standard of care for HER2-negative metastatic G/GEJ adenocarcinoma.
In terms of safety, the triple combination therapy exhibited a manageable profile, with common side effects such as nausea, hypoalbuminemia, and vomiting being mostly mild to moderate in severity. This suggests that the treatment is not only effective but also tolerable for patients undergoing therapy.
The results of the Phase II trial for Osemitamab (TST001) in combination with Nivolumab and CAPOX represent a significant advancement in the treatment landscape for patients with HER2-negative metastatic G/GEJ adenocarcinoma. With its promising efficacy and manageable safety profile, this triple therapy offers hope for individuals battling advanced gastric cancer, paving the way for improved outcomes and potentially setting a new standard of care in the field of oncology.













